

Given recent developments in India, where a dramatic and devastating second wave of COVID-19 infections started unexpectedly in early March, it is not surprising that other countries are concerned about the spread of variant B.1.617.2 and are discussing whether to either impose new COVID-19 restrictions or to delay further steps of easing re-strictions.
In this note we examine what is known about variant B.1.617.2. It does seem more transmissible than other vari-ants, but it doesn’t seem to significantly reduce the efficacy of existing vaccines as long as individuals are fully vaccinat-ed. Also, the morbidity and mortality associated with infec-tions with variant B.1.617.2 seem less than infections with variant B.1.1.7 (the variant first identified in Kent, England). This suggests that the most vulnerable individuals to variant B.1.617.2 are those that have not been fully vaccinated, which accounts for a significant proportion of the global pop-ulation, especially in Latin America, Asia, and Africa.
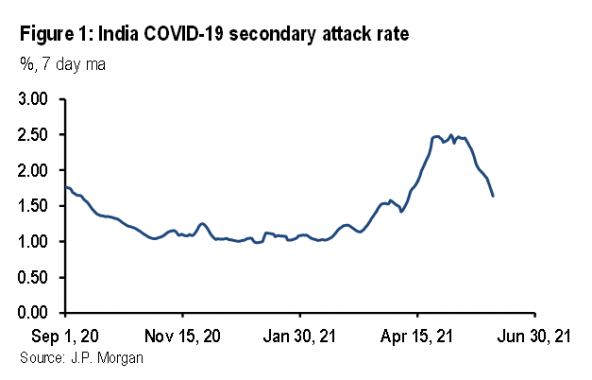
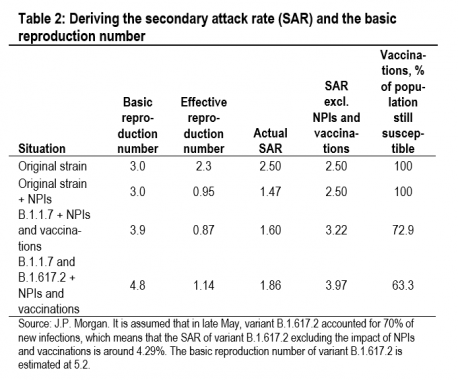
Table 2 presents our estimates of the impact of the SARS-CoV-2 variants in the UK using a framework where the SAR is calibrated from the reproduction number (estimated from daily infections), the average number of daily contacts (esti-mated from Google mobility data), and the number of days an infected individual is infectious (assumed to be eight throughout). At the start of the epidemic in the UK in mid-March 2020, the SAR of the original SARS-CoV-2 strain, ex-cluding the impact of non-pharmaceutical interventions (NPIs—social distancing, mask wearing, increased hygiene, and contact tracing and quarantine) and vaccinations, ap-peared to be around 2.5%. We have taken November 2020 as representative of a period with the original strain of SARS-CoV-2 with NPIs but before the impact of vaccinations (also with similar weather conditions to March). In November 2020 the actual SAR was around 1.47%. This suggests that NPIs reduced the SAR by 1.03%-pts, which is worth 1.2 on the reproduction number. By April 2021, the variant B.1.1.7 was dominant and the vaccination program was underway. The SAR ex-cluding the impact of NPIs and vaccinations looks to have been around 3.22%. By the time we get to late May, variant B.1.617.2 accounted for around 70% of new infections so the SAR of this variant excluding the impact of NPIs and vac-cinations looks to be around 4.29% (4.29=(3.97-(0.3*3.22))/0.7). These calculations suggest that variant B.1.1.7 is around 29% more infectious than the original strain of SARS-CoV-2, and that variant B.1.617.2 is around 33% more infectious than variant B.1.1.7. This suggests that variant B.1.617.2 is around 72% more infectious than the original strain.

These estimates of infectiousness based on UK data resonate with the reported data in India. Figure 1 shows the evolution of our estimate of the SAR in India. If we assume that the estimated SAR of 1.17% from September 2020 to January 2021 represented the original strain of SARS-CoV-2, and that the estimated SAR of 2.11% since April represents variant B.1.617.2, this suggests an increase in transmissibility of 80%, almost exactly the same as our estimate based on UK data. (Note that the estimated level of the SAR will not be the same across countries due to differences in demography, popula-tion density, and climate.)
The impact on the herd immunity threshold
Although our estimates of infectiousness based on the UK data are lower than those of PHE, they are still worrisome because it is the SAR excluding the impact of NPIs and vaccinations that determines the basic reproduction number,4 which determines the vaccination threshold for herd immunity.5 Our calculations suggest that it will be hard to reach herd immunity through vaccinations alone, and that some non-pharmaceutical interventions (NPIs) and mobility restrictions will likely need to remain in place for an extended period.
The calculations in Table 2 can be used to estimate the basic reproduction numbers for SARS-CoV-2 variants. These sug-gest that the basic reproduction number in the UK is 3.0 for the original strain, 3.9 for variant B.1.1.7, and 5.2 for variant B.1.617.2. These estimates for the basic reproduction number look high but they align with the estimates from the UK gov-ernment’s academic advisors (Table 3). On average, these academic institutions put the basic reproduction number for variant B.1.1.7 at 4.4. They have not yet published an esti-mate for variant B.1.617.2.
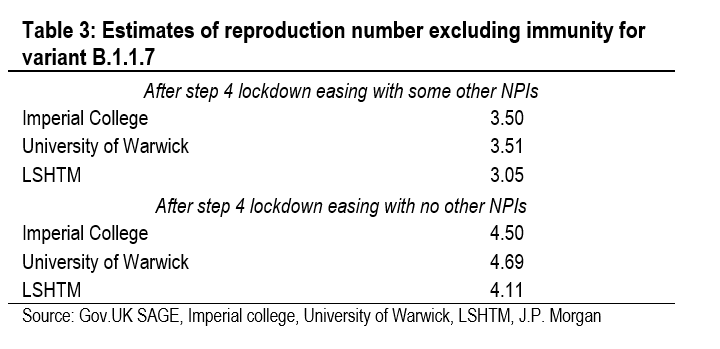
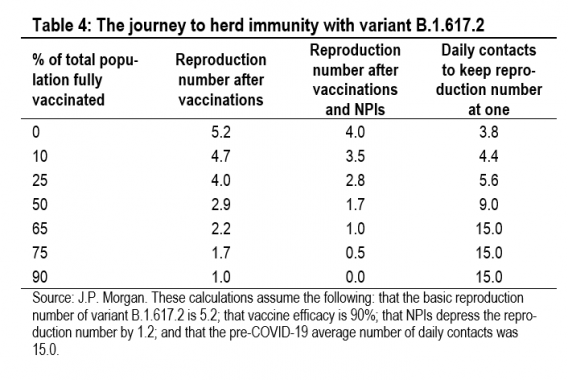
Table 4 illustrates what the journey to herd immunity looks like if the basic reproduction number of variant B.1.617.2 is 5.2. The second column shows the reproduction number after the impact of vaccinations but without any NPIs or mobility restrictions. With a fully susceptible population and no NPIs, the reproduction number is 5.2 (equal to the basic reproduc-tion number). Without NPIs, herd immunity is reached when 90% of the total population has been fully vaccinated (as-suming vaccine efficacy of 90%). At this level of vaccination, no NPIs are needed and daily contacts can return to the pre-COVID-19 average of 15. The third column shows the repro-duction number after the additional impact of NPIs, which are assumed to reduce the reproduction number by 1.2. If the current level of NPIs is maintained, then herd immunity is achieved once 65% of the total population is vaccinated. The final column shows what daily contacts have to do to get the reproduction number to one, given the impact of vaccinations and NPIs. With no vaccination effect but NPIs in place, daily contacts would need to be 3.8 (25% of the pre-COVID-19 average of 15) to hold the reproduction number at one. This broadly describes the UK in April 2020, when NPIs and daily contacts of 3.7 held the reproduction number at 1.1 (although the basic reproduction number at the time was 3.0).
Table 4 helps to understand the current situation in India. In early May, with very little protection afforded by the vaccine, the Indian reproduction number was brought down to one by a decline in daily contacts to 5.3. This is almost exactly what Table 4 would suggest, given our estimates for the basic re-production number and the impact of NPIs.
Variant B.1.617.2 and vaccine efficacy
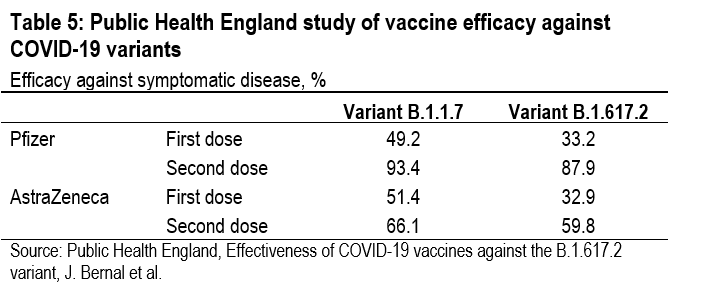
In a PHE study of vaccine efficacy against symptomatic disease, variant B.1.617.2 did significantly diminish the effi-cacy of the first dose of both the Pfizer-BioNTech and the Oxford-AstraZeneca vaccines, but not the efficacy of the second dose.6 After the first dose of the Pfizer-BioNTech vaccine, symptomatic infections were reduced by only 33.2% with the B.1.617.2 variant, while they were reduced by 49.2% with the B.1.1.7 variant. But after the second dose of the Pfizer-BioNTech vaccine, symptomatic infections were re-duced by 87.9% with the B.1.617.2 variant, compared with a reduction of 93.4% with the B.1.1.7 variant (Table 5). A simi-lar pattern is evident with the Oxford-AstraZeneca vaccine. These results explain why, after extending the gap between first and second doses to 12 weeks to make the limited supply go further, the UK government is now shortening the gap to provide more protection against variant B.1.617.2.
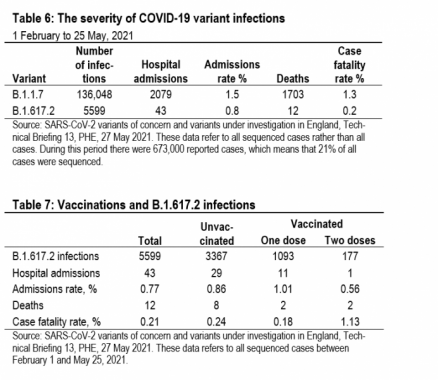
Vaccine efficacy is normally measured against symptomatic infections. But, that is not the only thing that matters. Vac-cines can also affect the morbidity and mortality of sympto-matic infections. Table 6 shows the results of a PHE study that looked at all sequenced cases between February 1 and May 25.7 Identified cases of infections with variant B.1.617.2 had a much lower hospital admissions rate and case fatality rate than infections with variant B.1.1.7.
A more detailed PHE study of vaccinations and variant B.1.617.2 presents a more nuanced picture (Table 7).8 Vac-cinated individuals are much less likely to become infected with variant B.1.617.2, especially if two doses have been ad-ministered. But when it comes to hospitalizations and deaths, it looks as if vaccinated individuals are more likely to be hos-pitalized and more likely to die than unvaccinated individuals (combining those with one and two doses). This seems im-plausible to us, given the other evidence, and most likely re-flects the very small sample sizes. Only 12 vaccinated indi-viduals were hospitalized and only 4 died.
Global vulnerabilities to SARS-CoV-2 vari-ant B.1.617.2
Our reading of the evidence suggests that variant B.1.617.2 is much more transmissible than either the original SARS-CoV-2 strain or variant B.1.1.7, but fully vaccinated individuals are well protected. This means that infections will spread very rapidly through a susceptible population, as has happened in India in recent months. And even though the hospitalization and case fatality rates might be lower with variant B.1.617.2 infections, a higher number of cases will still deliver an in-crease in the pressure on healthcare systems and more deaths. There is good reason for the world to be concerned about var-iant B.1.617.2.
What matters is the proportion of the total population that has been vaccinated, especially the proportion that has been fully vaccinated. Across Developed Markets only Japan, Aus-tralia, and New Zealand look especially vulnerable with vac-cination rates still relatively low. But the real concern is across Emerging Markets. In Latin America, vaccination rates are low everywhere except in Chile. In Africa, every country looks very vulnerable with very low vaccination rates across the whole continent. In Emerging Asia, everyone looks very vulnerable except for China and Singapore. Finally, in Emerg-ing Europe the most vulnerable countries are Russia and Tur-key. EU member states in Emerging Europe have benefited from the regional vaccine distribution scheme, while Israel is the least vulnerable country in the world with 60% of its total population fully vaccinated.
India’s experience in recent months illustrates what can hap-pen when SARS-CoV-2 variant B.1.617.2 runs through a sus-ceptible population. This argues strongly not only for faster vaccine production but also a more equal distribution. Of the 2.1 billion doses of vaccine administered thus far, 30% has gone to DM countries, even though they account for only 12% of the world’s population.
https://www.gisaid.org/hcov19-variants/
SARS-CoV-2 variants of concern and variants under investigation in England, Technical Briefing 13, Public Health England, 27 May 2021
Effectiveness of COVID-19 against the B.1.617.2 variant, J. Lopez Bernal.
SARS-CoV-2 variants of concern and variants under investigation in England, Technical Briefing 13, PHE, 27 May 2021.
SARS-CoV-2 variants of concern and variants under investigation in England, Technical Briefing 13, PHE, 27 May 2021.